Clinical research and development in China has grown rapidly in the past 5-10 years. However, a large number of developed drugs are trapped in homogeneous competition, and this situation will be difficult to sustain. As more and more companies focus on and attempt to address this issue, clinical trials in China are expected to transition from extensive growth to high-quality growth.
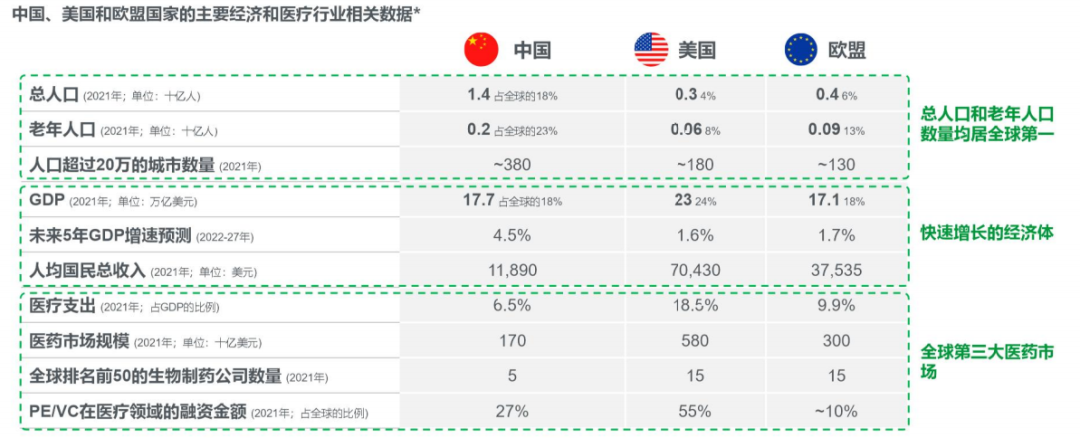
Basic Overview of Clinical Trials in China Based on the major economic and healthcare industry indicators of China, the United States, and EU countries, the Chinese healthcare market has a strong growth foundation. China currently has and will continue to have the largest patient population, and its ability to bear medical expenses is constantly increasing; However, at the same time, the Chinese market also faces many challenges such as pressure on drug prices, lack of patient awareness, and insufficient cutting-edge innovation.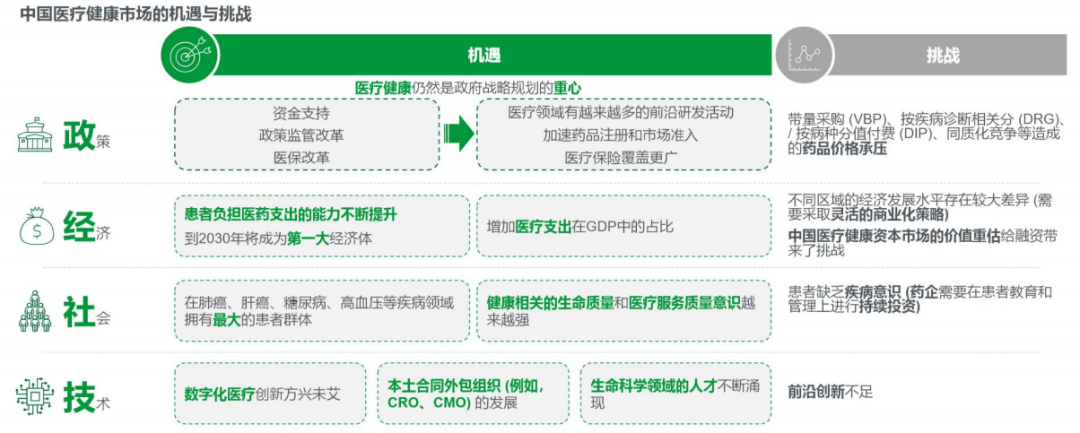
Driven by favorable policies, accelerated development of innovative drugs, and continuous increase in research and development investment, clinical trials in China have grown rapidly in the past five years;
Accelerated development of innovative drugs: China's early clinical trials have seen strong growth, and its innovation capabilities have quickly caught up with international leading levels. The development of new drugs has largely driven the strong growth in the number of clinical trials in China.
Between 2016 and 2021, the growth rate of early trials was faster, with a compound annual growth rate of 29% and 47% for Phase I and II trials, respectively;
In certain innovative fields, China has become a global leader in clinical development, such as CAR-T and ADC.
2. Future Development Trends of Clinical Trials in China
Despite facing many challenges, the foundation of China's healthcare market remains very solid and is expected to support high-quality growth in clinical trials in the future. In this context, four major strategic trends can be foreseen:
1) Developing more innovative and differentiated therapies: In recent years, Chinese pharmaceutical companies have made certain progress in innovation; However, many drug developments are focused on popular targets such as PD-1 and EGFR, leading to homogeneous competition. As pharmaceutical companies begin to focus on new drug models, technology platforms, and targets, it is expected that more innovative and differentiated therapies will emerge. At the same time, in order to cope with homogeneous competition, pharmaceutical companies are also focusing on more emerging drug models, such as bispecific antibodies, antibody conjugated drugs, and cell/gene therapies, which will make the Chinese market more diversified.
2) More and more attention is being paid to research and development that is more commercially attractive. In the past few years, cancer has become the hottest treatment field in clinical development in China. In the future, pharmaceutical companies will often consider pharmaceutical research and development more comprehensively, and the commercial attractiveness of products will become a crucial factor to consider. Other considerations include investment return rate, the company's own resource endowment, and the feasibility of external cooperation.
With the launch of more innovative products, the focus of market attention has shifted from the previous "rapid promotion of successful product launch" to having "commercial appeal", as it directly determines whether a product can ultimately succeed in increasingly homogeneous competition.
More importantly, products with commercial appeal can better ensure the sustainable development of the company in the future:
Sustainable market revenue for future innovative research and development;
Strong valuation to attract future external investment;
The successful transformation from a biotechnology company to a biopharmaceutical company.
3) The application of more intelligent clinical trial tools: With the increasing complexity of clinical trials, the demand for intelligent and digital tools is increasing day by day. Technologies such as Electronic Data Collection System (EDC), Clinical Trial Project Management System (CTMS), Clinical Trial Master Document Management System (eTMF), and Pharmacovigilance System (PVS) have been widely used in clinical trials in China. I believe that in future clinical trials, intelligent and digital tools will become indispensable, and even bring revolutionary impacts.
Remote clinical trials have become a hot topic and have recently received attention from regulatory authorities. The future development of remote clinical trials faces both driving forces and obstacles. Improving clinical trial efficiency is the main driving force, while policy uncertainty is the main obstacle.
4) The globalization of Chinese biopharmaceutical companies and the continuous evolution of research and development strategies of multinational pharmaceutical companies in China: Globalization is becoming increasingly important for Chinese biopharmaceutical companies. In order to successfully carry out overseas clinical trials, more and more pharmaceutical companies are considering factors such as carefully selecting destination countries, establishing research and development teams locally, and actively communicating with local regulatory agencies; Local innovative drugs are gaining recognition in the international market, and their global interests are constantly being acquired by overseas enterprises;
Among them, leading multinational pharmaceutical companies are actively authorizing the introduction of innovative assets from China. Multinational pharmaceutical companies are shifting from establishing Chinese R&D centers to establishing Chinese partner centers or incubation centers to promote cooperation with local biopharmaceutical companies in China.
相关推荐

