Recently, the general office of Guangdong Provincial People's government issued the implementation plan for promoting the high-quality development of medical device industry in Guangdong Province, which defined the guiding ideology and development objectives of promoting the high-quality development of medical device industry in Guangdong Province, and put forward eight main tasks and three safeguard measures to promote the high-quality development of medical device industry. The plan closely adheres to the theme of promoting high-quality development, strengthens planning guidance and policy support, strengthens original and leading scientific and technological research, constructs an innovation ecology of medical devices with innovation leading industrial development, strives to break through key core technologies and enhance the independent and controllable ability of key parts and key raw materials, Promote the supervision ability of medical devices in our province to take the lead in reaching the international advanced level.
Notice of the general office of the people's Government of Guangdong Province on printing and distributing
Promote the high-quality development of medical device industry
Notice of implementation plan
Ybh [2021] No. 366
Municipal People's governments at or above the local level, departments and institutions directly under the provincial government:
With the consent of the provincial people's government, the implementation plan of Guangdong Province for promoting the high-quality development of medical device industry is hereby printed and distributed to you. Please earnestly implement it in combination with the actual situation. Please report the problems encountered in the implementation to the provincial food and drug administration.
General Office of provincial government
December 31, 2021
Guangdong province promotes high-quality medical device industry
Development implementation plan
1、 General requirements
(1) Guiding ideology.
Promote high-quality development, deeply implement the innovation driven development strategy, give full play to the advantages of the medical device industry in our province, highlight the focus, supplement the shortcomings, improve the innovation ecology, strive to realize the integration of the industrial chain and the promotion of the value chain, and realize the innovative development of medical devices.
(2) Development goals.
Closely follow the theme of promoting high-quality development, strengthen planning guidance and policy support, strengthen original and leading scientific and technological research, build a medical device innovation ecosystem with innovation leading industrial development, strive to break through key core technologies, enhance the independent and controllable ability of key parts and components and key raw materials, and promote the medical device supervision ability of our province to take the lead in reaching the international advanced level.
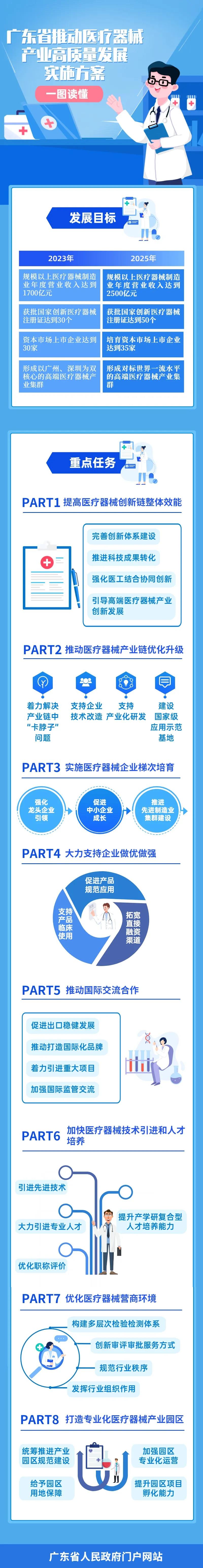
Strive to achieve a compound annual growth rate of more than 20% in the operating revenue of the medical device manufacturing industry by 2023, and the annual operating revenue of the medical device manufacturing industry above Designated Size will reach 170 billion yuan; 30 national innovative medical device registration certificates have been approved; 30 listed enterprises in the capital market; Form a high-end medical device industry cluster with Guangzhou and Shenzhen as the dual core.
Strive to achieve an average annual compound growth rate of more than 20% of the operating revenue of the medical device manufacturing industry by 2025, and the annual operating revenue of the medical device manufacturing industry above Designated Size will reach 250 billion yuan; 50 national innovative medical device registration certificates have been approved; Cultivate 35 listed enterprises in the capital market, 2-3 demonstration enterprises with a market value of more than 100 billion yuan, 3-5 leading enterprises with an annual operating income of more than 10 billion yuan and 5-8 leading enterprises with an annual operating income of more than 5 billion yuan; Build a number of backbone enterprises with independent brands with international influence and form a world-class high-end medical device industry cluster.
2、 Key tasks
(1) Improve the overall efficiency of the medical device innovation chain.
1. Improve the construction of innovation system. Build a medical device technology innovation system with enterprises as the main body, market-oriented and deep integration of industry, University and research. Vigorously improve the source innovation ability of high-end medical devices, focus on cutting-edge technology fields, and accelerate the research on key core technologies and applications in the fields of high-end medical imaging and advanced treatment equipment, high-end in vitro diagnostic instruments and reagents, high-end implantation (Introduction) devices and biomedical materials. Optimize and innovate the linkage working mechanism of research and examination of medical devices, optimize communication methods and channels, and strengthen technical guidance and consulting services for applicants. (led by the Provincial Department of science and technology and responsible by the provincial food and drug administration according to the division of responsibilities)
2. Promote the transformation of scientific and technological achievements. Support the establishment of a platform for the transformation of medical achievements, and promote the industrialization of medical achievements with the help of the platform in disciplines, talents, projects and transformation. Support medical device R & D and transformation institutions to undertake research achievements with significant application prospects and innovation in Colleges and universities, scientific research institutes and medical institutions. Accelerate the implementation and application of new technologies and products of medical devices. Establish a reasonable distribution mechanism of responsibilities and rights of all participants in the transformation of achievements. Strengthen the protection of intellectual property rights of medical devices, and establish a collaborative protection system of rapid patent examination, rapid confirmation of rights and rapid protection of rights; Timely provide trademark registration, intellectual property analysis and evaluation, intellectual property quick financing and loan services, and promote intellectual property transactions. (led by the Provincial Department of science and technology, the Provincial Department of education, the Health Commission, the market supervision bureau and the Municipal People's governments at and above the prefecture level are responsible according to the division of responsibilities)
3. Strengthen the combination of medicine and industry and collaborative innovation. Support medical and health institutions and clinicians to participate in technological innovation of medical devices and actively carry out collaborative research and development of medical devices; Bring the construction of clinical trial institutions into the important content of the construction and development of medical and health institutions, establish the working mechanism of clinical trial ethics cooperation and review, explore the incentive mechanism for the implementation of clinical trial institutions and clinical trial implementation medical personnel, and promote the innovation of medical device industry. (under the leadership of the provincial health and Health Commission, the provincial food and Drug Administration and the Municipal People's governments at or above the prefecture level are responsible according to the division of responsibilities)
4. Guide the innovation and development of high-end medical device industry. Cultivate a number of major industrial projects in key fields such as biomedical materials, implant (Introduction) devices, gene testing, surgical robots, artificial intelligence medical devices and medical endoscopes. Support the leading enterprises in the subdivided fields of our province to build a national manufacturing innovation center. Give full play to the advantages of Dawan sub center for technical evaluation and inspection of medical devices of the State Drug Administration, and promote the leading and radiating role of Dawan District in medical device innovation. (led by the provincial development and Reform Commission, the Provincial Department of industry and information technology, the food and Drug Administration and the Municipal People's governments at and above the prefecture level are responsible according to the division of responsibilities)
(2) Promote the optimization and upgrading of the medical device industry chain.
5. Strive to solve the problem of "neck sticking" in the industrial chain. Layout and strengthen the capacity-building of key materials, core parts (components) and advanced technology. Support medical device enterprises and upstream enterprises in cross industry collaborative technology research, and upstream enterprises enjoy the same policy treatment. Supplement and strengthen the short board in the fields related to medical devices. The medical device registration matters involved shall be included in the scope of priority review and approval services. (led by the Provincial Department of science and technology and the Department of industry and information technology, the provincial development and Reform Commission, the food and Drug Administration and the Municipal People's governments above the prefecture level are responsible according to the division of responsibilities)
6. Support the technological transformation of enterprises. Increase support for the technological transformation of medical device enterprises, encourage enterprises to adopt applicable new technologies, new processes, new equipment and new materials for technological transformation, promote enterprises to expand investment in equipment renewal and technological transformation, and give a certain proportion of support to qualified technological transformation projects of manufacturing enterprises according to their equipment purchase amount and technological transformation investment amount. Strengthen the research and support for the intelligent manufacturing level of the medical device industry. (led by the Provincial Department of industry and information technology, the Provincial Department of Finance and municipal people's governments above prefecture level are responsible according to the division of responsibilities)
7. Support industrial R & D. Support leading enterprises in the subdivided field of medical device industry in our province to build product R & D centers. Accelerate the R & D and industrialization of relevant fields, improve the production process and product quality, and improve the development level of medical device industrialization. Strengthen the research and development of advanced TCM medical devices and promote the modernization of TCM medical devices. (led by the Provincial Department of science and technology and the Department of industry and information technology, the Provincial Health Commission, the food and Drug Administration and the Municipal People's governments above the prefecture level are responsible according to the division of responsibilities)
8. Construction of national demonstration base. Promote the construction of demonstration and Application Center for health care big data and domestic innovative medical device products. Relying on the advanced medical equipment application demonstration project and 5g + medical and health application pilot project of the Ministry of industry and information technology and the National Health Commission, support relevant enterprises and medical institutions to establish demonstration bases with regional radiation influence, form a benign mechanism of R & D Application feedback improvement promotion, and help enterprises upgrade their products iteratively. (led by the provincial development and Reform Commission and the health and Health Commission, the Provincial Department of industry and information technology and the food and drug administration are responsible according to the division of responsibilities)
(3) Implement echelon cultivation of medical device enterprises.
9. Strengthen the leadership of leading enterprises. Leading enterprises are encouraged to focus on the key links and core technologies of the industrial chain, carry out joint ventures, mergers and acquisitions, integrate resources, complement each other's advantages, strengthen brand cultivation, and constantly improve their core competitiveness and industry driving force. Optimize services, include medical device products undertaking industrial transfer into the scope of priority review and approval services, and simplify the requirements for application materials of transferred products. (led by the Provincial Department of industry and information technology, the provincial market supervision bureau and the drug administration are responsible according to the division of responsibilities)
10. Promote the growth of small and medium-sized enterprises. We will guide small and medium-sized medical device enterprises to focus on key core technologies, improve their innovation ability and market share, and take the development path of "specialization and innovation". Cultivate a number of "little giant" enterprises in the subdivided field of medical devices and single champion enterprises in manufacturing industry, which will be included in the scope of priority review and approval services. (led by the Provincial Department of industry and information technology and the food and drug administration, and the provincial market supervision bureau is responsible according to the division of responsibilities)
11. Promote the construction of advanced manufacturing clusters. Enhance the leading role of leading and backbone enterprises, strengthen professional cooperation and supporting capacity, and further promote the development of industrial clusters. Support cluster promotion institutions, vigorously promote the construction of national advanced manufacturing clusters of "Shenzhen Guangzhou high-end medical devices", and promote the agglomeration of advantageous resource elements and cross industry coordination. Support the construction of common technology R & D platform and public service platform. Strengthen the coordination and guidance of advanced manufacturing cluster projects. (led by the Provincial Department of science and technology and the Department of industry and information technology, the provincial food and Drug Administration and the Municipal People's governments above prefecture level are responsible according to the division of responsibilities)
(4) Vigorously support enterprises to become better and stronger.
12. Promote the standardized application of products. Accelerate the clinical promotion and use of innovative medical devices. We will improve medical service prices and medical insurance payment policies, dynamically adjust the catalogue of medical consumables and medical service prices, timely include new products of medical consumables into the scope of medical insurance payment according to regulations, improve the coordination mechanism between medical insurance payment standards and centralized purchase prices, and actively promote the direct settlement between medical insurance funds and medical consumables enterprises. Strengthen the construction of intelligent supervision information system for medical devices, improve the data information sharing mechanism, and improve the efficiency of bidding and procurement. (led by the provincial medical insurance bureau, the Provincial Department of finance, the health and Health Commission and the food and drug administration are responsible according to their respective responsibilities)
13. Support the clinical use of the product. Medical institutions are encouraged to increase the procurement and use of domestic medical devices with accurate efficacy, controllable quality and stable supply. Support the production of the first set of high-end medical equipment in our province, and implement the requirements of relevant documents of the first set of domestic equipment for the large-scale medical equipment listed in the promotion and Application Guidance Catalogue of the first set of major technical equipment settled and produced in our province. (led by the provincial health and Health Commission, the Provincial Department of finance, the medical insurance bureau and the food and drug administration are responsible according to the division of responsibilities)
14. Broaden direct financing channels. We will continue to promote the listing and financing of high-quality enterprises in domestic and foreign securities markets, and support qualified enterprises to expand direct financing channels through equity financing, corporate credit bonds and asset securitization. Guide all kinds of equity investment institutions, private equity investment funds and venture capital funds to invest in the medical device industry. Improve the investment of government guided funds in the seed stage and angel stage of enterprises, and encourage teams with medical industry background or industrial companies to establish venture capital funds, angel funds and other early medical industry sub funds. Listed companies, companies listed on the new third board and companies to be listed that have completed counseling and filing will be included in the scope of priority review and approval services. (led by the Provincial Local Financial Supervision Bureau, the provincial development and Reform Commission, the Department of industry and information technology, the food and drug administration, the Guangdong securities regulatory bureau and the Shenzhen Securities Regulatory Bureau are responsible according to the division of responsibilities)
(5) Promote international exchanges and cooperation.
15. Promote the steady development of exports. Implement and improve export tax rebate and export credit policies, and support the export of medical device products. Encourage the promotion of new marketing formats such as online exhibition and sales, and improve the supervision mechanism suitable for the healthy development of new marketing formats. Encourage enterprises to participate in overseas medical device exhibitions and academic conferences, and support the holding of international medical device exhibitions and academic conferences. (led by the Provincial Department of Commerce, the provincial development and Reform Commission, the market supervision bureau, the provincial taxation bureau, the Guangzhou Branch of the people's Bank of China and the Municipal People's governments above the prefecture level are responsible according to the division of responsibilities)
16. Promote the building of international brands. Support independent brand medical device enterprises to explore the international market, encourage enterprises to set up overseas R & D centers and production bases, and accelerate the global layout. Establish a comprehensive service platform for trademarks and brands, support independent trademark enterprises to implement trademark strategy, support enterprises to actively register overseas trademarks, and expand the popularity and influence of independent brands. (led by the provincial development and Reform Commission and the Department of Commerce, the provincial market supervision bureau and the Municipal People's governments above prefecture level are responsible according to the division of responsibilities)
17. Focus on introducing major projects. Strengthen project cooperation, technology and talent exchange, and actively introduce major international medical device industry projects; Encourage domestic enterprises to cooperate with overseas advanced enterprises and scientific research institutions; Introduce large overseas enterprise groups, large projects in the high-end and key links of the industry and advanced manufacturing technology to our province; Attract internationally renowned multinational companies and scientific research institutions to set up headquarters and R & D centers in our province. All localities are encouraged to implement "one enterprise, one policy" and one-stop service for key introduced projects and enterprises. (led by the Provincial Department of Commerce, the provincial development and Reform Commission, the Department of industry and information technology and the Municipal People's governments at and above the prefecture level are responsible according to the division of responsibilities)
18. Strengthen international regulatory exchanges. Learn from international advanced management experience, strengthen the research on international regulatory regulations, promote the overseas on-site inspection of imported key parts and components and key raw materials for the production of medical devices, and ensure the quality and safety of medical devices; Strengthen the research on international medical device standards, promote the integration with international standards and improve the technical support capacity of standards; Registrants of imported medical devices are encouraged to produce medical device products in our province through their foreign-invested enterprises established in China. (led by the provincial food and drug administration, the Foreign Affairs Office of the provincial Party committee, the Provincial Department of Finance and the Department of commerce are responsible according to the division of responsibilities)
(6) Accelerate the introduction of medical device technology and personnel training.
19. Introduce advanced technology. Aiming at the cutting-edge fields and top level of medical devices in the world, introduce and absorb advanced technologies at home and abroad, and focus on the introduction of strategic talents and leading talents and teams who can break through key technologies, develop high-tech industries and drive emerging disciplines. (led by the Provincial Department of science and technology, the Provincial Department of industry and information technology and the Municipal People's governments above prefecture level are responsible according to the division of responsibilities)
20. Vigorously introduce professionals. Support institutions of higher learning and enterprises to introduce high-level talents in the field of medical devices, and relax the conditions for employment and entrepreneurship in Guangdong. Provide convenience measures for high-end talents to apply for work permits and work residence permits; Simplify the examination procedures for talents who have obtained the work permit of high-end talents (Class A) in Beijing and Shanghai to work in our province, and improve the introduction and retention of high-end talents. (led by the Provincial Department of science and technology and responsible by the Provincial Department of education according to the division of responsibilities)
21. Improve the training ability of industry university research talents. Improve the mode of industry university research cooperation to cultivate innovative talents, rely on Colleges and universities and scientific research institutes, promote the cross integration of medical disciplines and engineering disciplines, and transport technical talents to the industry; Deepen school enterprise cooperation, innovate talent training mode, encourage qualified enterprises to set up postdoctoral scientific research platforms or teaching and training bases, and promote the organic connection between education chain, talent chain, industrial chain and innovation chain; Break down institutional barriers and further open up the circulation channels of high-level technical talents in Colleges and universities, scientific research institutes and enterprises; Support enterprises to carry out the training of compound medical and engineering talents. (led by the Provincial Department of education, the Provincial Department of science and technology, the Department of industry and information technology and the Department of human resources and social security are responsible according to the division of responsibilities)
22. Optimize professional title evaluation. Optimize the professional title evaluation of engineering and technical talents of medical devices, encourage high-level professional and technical talents to actively participate in the professional title evaluation, evaluate applied talents with strong practicality and operability and unclear research attributes, break the paper only and award only standards, and set up reasonable assessment indicators in line with the actual situation of the post. (led by the provincial food and Drug Administration and in the charge of the provincial human resources and social security department according to the division of responsibilities)
(7) Optimize the business environment of medical devices.
23. Build a multi-level inspection and detection system. Encourage enterprises to build R & D registered self inspection laboratories to improve their self inspection ability; Build a diversified third-party inspection and testing platform that complements each other and has its own characteristics; Strengthen the capacity-building of legal inspection and testing technical support institutions, strengthen their public welfare attribute, technical authority attribute and technical service attribute, and continuously shorten the inspection and testing time. (led by the provincial food and Drug Administration and responsible by the provincial market supervision bureau according to the division of responsibilities)
24. Innovate the service mode of review and approval. Explore the reform of medical device registrant system in Dawan District, Guangdong, Hong Kong and Macao. Implement the information management of the whole process of review and approval, continuously improve the review ability and efficiency, optimize the review and approval process, establish a filing and review system, improve the communication mechanism of registration correction data, establish a review and approval efficiency evaluation system from the registration acceptance date to the final issuance date, and actively promote the shortening of the time limit of technical review and the registration cycle of class II medical devices. (led by the provincial food and Drug Administration and responsible by the provincial market supervision bureau according to the division of responsibilities)
25. Regulate industry order. Continue to strengthen the improvement of supervision ability, innovate supervision mechanism, strengthen the coordinated supervision of provinces, cities and counties, and form a chess pattern of supervision in the whole province. Strengthen the linkage of provinces, cities and counties in inspection and testing, review and approval, daily supervision, inspection and case handling, monitoring and evaluation, etc. Improve the hierarchical and classified supervision mechanism based on enterprise credit and product risk, make full use of information technology, promote the digital supervision of medical devices in the whole life cycle, promote inclusive and prudent supervision, and severely crack down on illegal production, operation and use, so as to form a supervision ecology more conducive to the high-quality development of the industry. (led by the provincial food and Drug Administration and responsible by the provincial market supervision bureau according to the division of responsibilities)
26. Give full play to the role of industry organizations. Support industry organizations to take the lead in establishing industrial alliances in different fields, and encourage enterprises to participate in the formulation and revision of national and industrial standards. Support industry organizations to carry out data collection, monitoring and analysis of industrial development, form various analysis reports, and give full play to the service role of industry organizations in policy formulation, industrial guidance, introduction of major projects, skilled personnel training, regional brands, construction of industrial parks, etc. Guide industry organizations to establish a supply chain procurement platform, improve the negotiation ability of "neck" products, and encourage enterprises to purchase key parts and raw materials through the platform. (led by the provincial food and drug administration, the Provincial Department of industry and information technology, the Department of Commerce, the market supervision bureau and the Municipal People's governments above prefecture level are responsible according to the division of responsibilities)
(8) Build a professional medical device industrial park.
27. Comprehensively promote the standardized construction of industrial parks. Support policies for investment attraction, R & D and innovation, design and manufacturing, brand building, exhibitions and trade shows, intellectual property rights, talent training, etc. will be implemented in the park, create a resource sharing platform for the medical device industry chain, attract innovative, leading and complementary R & D platforms, and promote the transformation and implementation of high-quality medical device achievements in our province. (led by the provincial development and Reform Commission and the Department of industry and information technology, the Provincial Department of human resources and social security, the Department of Commerce, the market supervision bureau and the Municipal People's governments above the prefecture level are responsible according to the division of responsibilities)
28. Provide land security for the park. Regions with conditions are encouraged to incorporate the construction of professional industrial parks of medical devices into the land and space planning, and strengthen the supply of special land. For areas that use the "three old" transformation land for scientific and technological innovation projects in the medical device industry, the planned indicators of new construction land shall be rewarded in accordance with the relevant provisions of the province. Differential land security can be provided by leasing before transfer and flexible term transfer. (led by the Provincial Department of natural resources and responsible by the Provincial Department of industry and information technology according to the division of responsibilities)
29. Strengthen the professional operation of the park. With the professional park as the core, gather the resource elements of industrial development, improve the efficiency of resource allocation, and promote the common technology platform and public service platform to settle in the professional park. All localities explore and implement the "key Park assignment system", implement special personnel, provide policy and technical services, and support the differentiated and large-scale development of professional parks. (under the leadership of Municipal People's governments at or above the local level, the provincial development and Reform Commission, the Department of industry and information technology, the Department of human resources and social security, the Department of Commerce, the market supervision bureau and the food and drug administration are responsible according to the division of responsibilities)
30. Improve the project incubation capacity of the park. Guide the construction of professional incubators, introduce professional operation institutions and professional public technology platforms; Adopt the combination of external introduction and internal fission to support listed enterprises and leading enterprises in subdivided fields to build incubators by using their own advantages; Support specialized investment institutions to centrally incubate invested projects, and local relevant departments and industry organizations to provide professional guidance. (under the leadership of Municipal People's governments at or above the local level, the Provincial Department of science and technology, the Department of industry and information technology and the Department of human resources and social security are responsible according to the division of responsibilities)
3、 Safeguard measures
(1) Strengthen organizational leadership. *** Relying on the promotion mechanism of the "chain leader system" of the biomedicine and health industry cluster of the provincial government, coordinate the overall work of the high-quality development of the medical device industry in our province, strengthen strategic planning and resource planning, and coordinate various departments to establish a linkage, coordination and promotion mechanism. All localities shall establish and improve the work coordination mechanism of multi sectoral linkage, study major matters related to industrial development, and coordinate and solve major problems in the promotion.
(2) Increase policy support. Implement the policy of helping enterprises and benefiting enterprises in detail, coordinate relevant special funds, increase support for medical device enterprises, form a financial support mechanism dominated by cities and linked by provinces and cities, implement various preferential tax policies to support the development of medical device industry, and focus on ensuring the addition and deduction of R & D expenses incurred by medical device enterprises The pre tax deduction policies for marketing and market access expenses were fully implemented. Support the local hosting of key exhibitions and forums and the early medical industry sub fund according to regulations.
(3) Strengthen the implementation of responsibilities. All leading departments should focus on the objectives and tasks, formulate supporting policies and measures according to the division of functions, establish special work accounts, and fully implement key tasks. All localities should formulate comprehensive supporting policies and plans in combination with the actual situation of industrial development, promote the formation of a systematic and provincial linkage policy system to promote the high-quality development of the medical device industry, accelerate the implementation of policies and ensure the achievement of the expected development goals.
Source: Official Website of Guangdong Provincial People's Government
相关推荐

