Company Introduction:
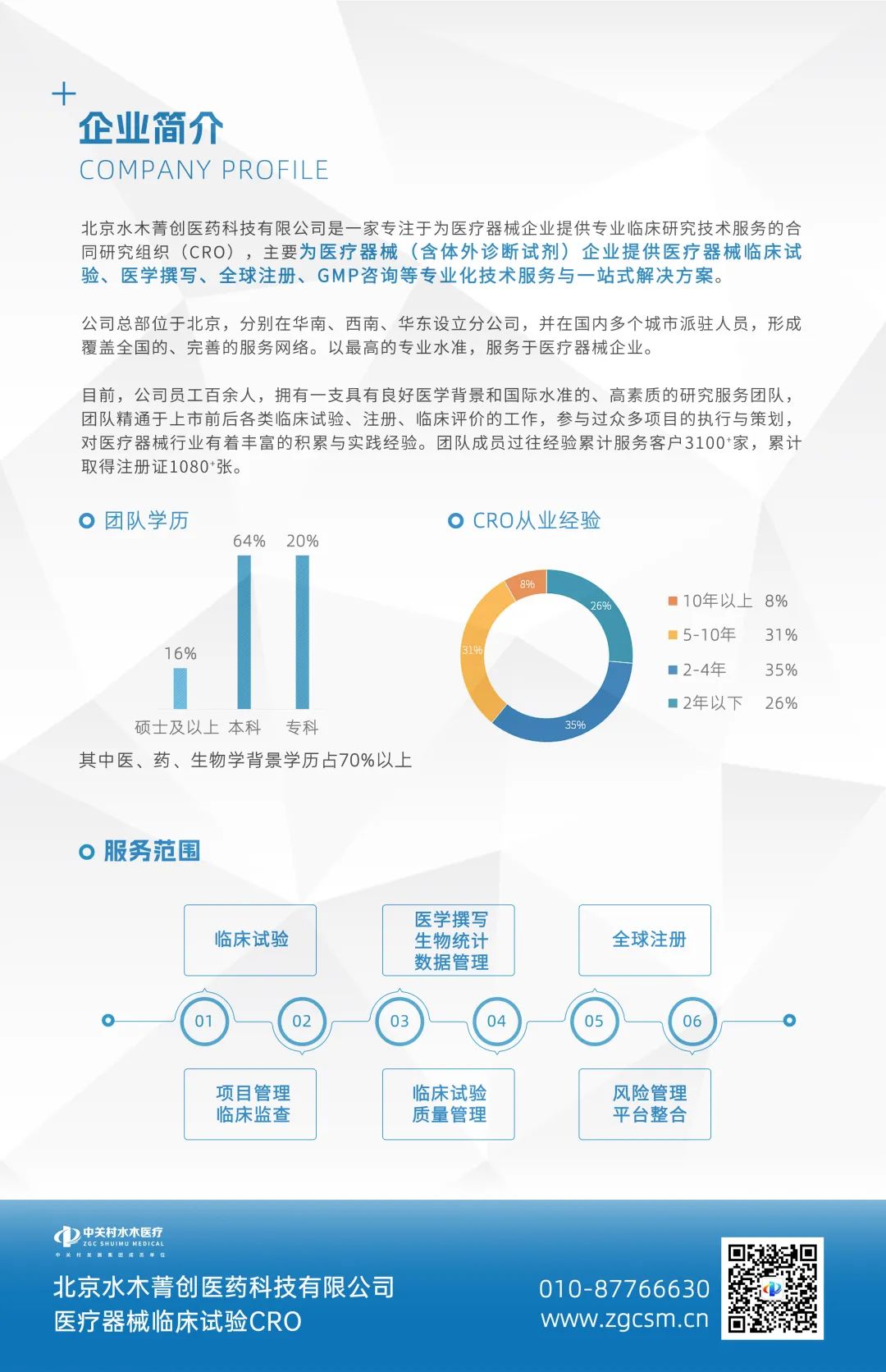
Beijing Shuimu Jingchuang Pharmaceutical Technology Co., Ltd. is a contract research organization (CRO) that focuses on providing professional clinical research technical services for medical device enterprises. It mainly provides professional technical services and one-stop solutions for medical device (including in vitro diagnostic reagents) enterprises, including clinical trials, medical writing, global registration, GMP consulting, and more. The company is headquartered in Beijing and has established branches in South China, Southwest China, and East China, with personnel stationed in multiple cities in China, forming a comprehensive service network covering the whole country. Serve medical device enterprises with the highest professional standards. At present, the company has more than 100 employees and a high-quality research and service team with a good medical background and international standards. The team is proficient in various clinical trials, registration, and clinical evaluations before and after listing, has participated in the execution and planning of numerous projects, and has rich accumulation and practical experience in the medical device industry.
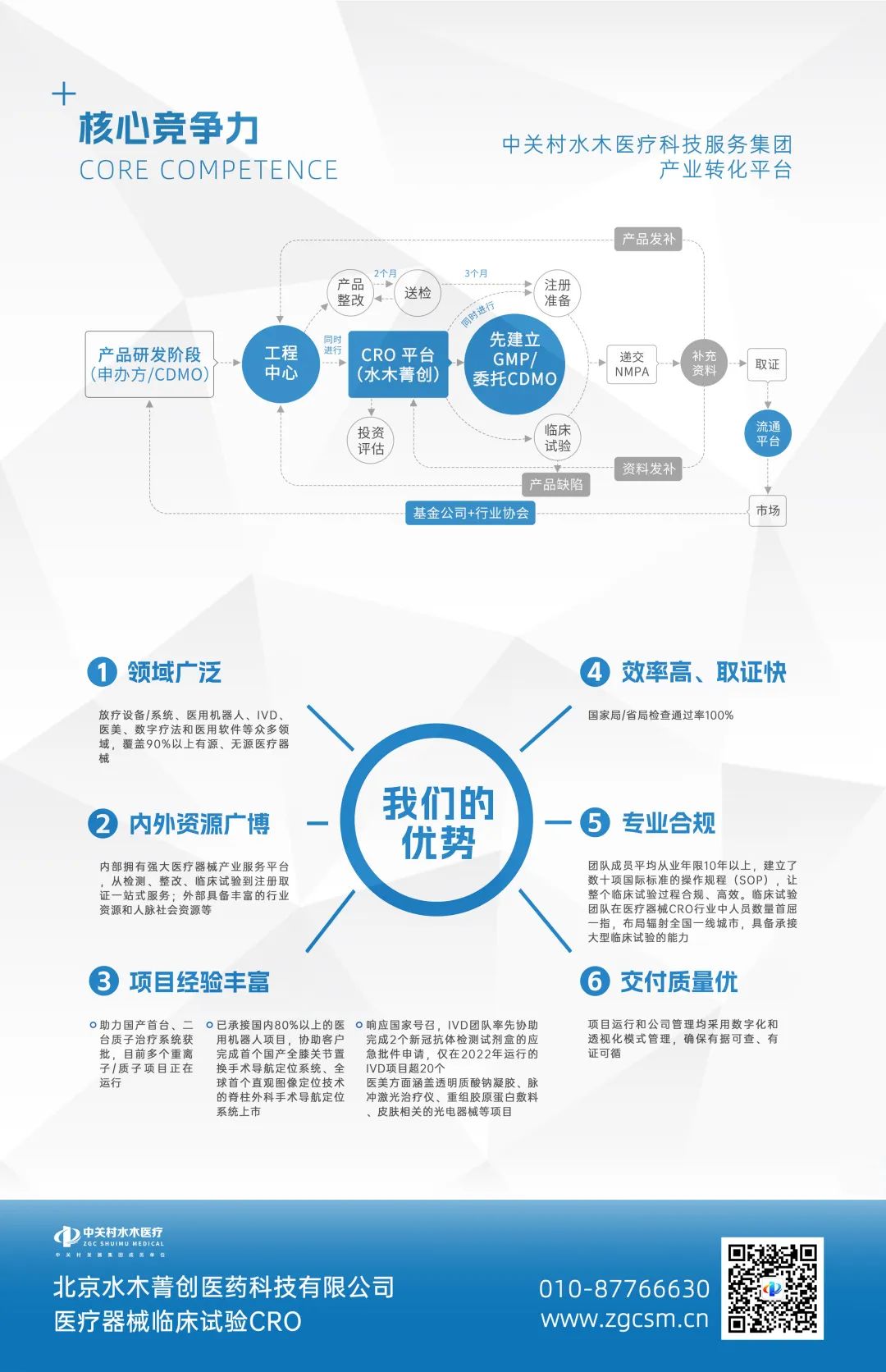
Shuimu Jingchuang's advantages lie in many fields such as radiotherapy equipment/systems, medical robots, IVD, medical aesthetics, digital therapy, and medical software. Mainly focusing on innovative and domestically produced medical device products, covering almost all categories such as active, passive, and IVD. There are currently over 100 ongoing projects, and team members have accumulated over a thousand past project experiences. Timely and high-quality project delivery; High efficiency and fast evidence collection; The national/provincial inspection pass rate is 100%. In 2022 alone, we assisted clients in obtaining 15 registration certificates.
Since its establishment, the company has always adhered to the values of "doing well, daring, diligent, and promising", providing professional support for China's medical device research, testing, production, and business, and building a comprehensive medical device lifecycle service platform. Our professional and efficient services have been recognized by many cooperative enterprises. With strict and effective management systems and a proactive purpose, we have obtained a series of authoritative certifications, including software copyright registration certificates, ISO quality management system certification, and the Zhongguancun National Independent Innovation Demonstration Zone high-precision and cutting-edge industrial innovation platform.
Business Introduction: