On December 7, 2025, the conference on high-quality development of innovative drugs was held in Guangzhou, which was the first time that China left Beijing to publish the catalogue of the year in the eight years since the medical insurance negotiations.
From spring to winter, the first edition of the Catalogue of Innovative Drugs for Commercial Health Insurance, which has been hotly debated and expected by the industry for a whole year, has finally been unveiled: from the initial 121 high-value innovative drugs that passed the formal review, only 19 of them were successfully screened.Roughly speaking, Weicai and Lilly are two AD drugs; All five declared million CAR-T therapies were shortlisted; A number of rare disease drugs were included; Domestic forces such as Baekje, Beihai Kangcheng, and Luye also occupied a successful position.In recognition of the first batch of participants, Director Zhang Ke of the Medical Insurance Bureau presented awards to them one by one. It is both an affirmation and a clear policy orientatio
▲ The first batch of enterprises short-listed in the catalogue of innovative drugs for commercial insurance took the stage to receive awards.
In terms of the basic medical insurance catalogue, it is also "full of faces". 114 new varieties were read out one by one by Huang Xinyu, director of the Department of Medicine Management of the National Medical Insurance Bureau. Such a sense of ceremony has never been seen before
▲ Read out the list of new varieties one by one on site.
The era of innovative drugs in China has indeed come.
By the end of October, the total transaction volume of domestic innovative drug BD in the sea exceeded 100 billion US dollars, and the down payment reached 8.1 billion US dollars, both exceeding the whole year of last year. Medical insurance is a key role in promoting this cage change-eight years of negotiations, which has driven the sales of innovative drugs in the catalogue to exceed 600 billion.
However, in comparison, everything in the catalogue of innovative drugs for commercial health insurance is still in its infancy-in 2024, Huimin Insurance paid only 1.1 billion yuan for innovative drugs, which is still a drop in the bucket in the nearly trillion-scale track.
At the meeting, the leaders of Guangdong Province, National Health Commission, the Food and Drug Administration, the National Development and Reform Commission, and the heads of the Administration of Traditional Chinese Medicine delivered speeches in turn; But on the other hand, the representatives of the insurance industry who were present but didn't speak out conveyed another subtle emotion of the construction of this "multi-level security system" in silence.
An era story about how innovative drugs, medical insurance and commercial insurance can build a new pattern has just begun.
01
The "frustrated" behind the highlights
The first signal of medical insurance negotiation in 2025 is that the status of innovative drugs in "China medical insurance system" has undergone a structural leap.
This year, 114 kinds of drugs were added, of which 50 kinds were innovative drugs, accounting for 44%, setting a record over the years.
On the surface, it is "the year of innovative medicine"; But deeper, this is a "survivor list" with high threshold, strong competition and refined screening.
From the data, we can see this differentiation between cold and warm: this year, a total of 534 drugs were submitted for preliminary screening, and only 114 drugs finally entered the catalogue, with a elimination rate as high as 79%.
Compared with previous years:
2024: 91 new drugs were added, of which 38 were innovative drugs, accounting for 41.7%.
2023: 121 new drugs and 23 first-class innovative drugs were added, and the negotiation or bidding was successful, accounting for 19%.
On the approval side, Yang Sheng, deputy director of National Medical Products Administration, gave a key background: as of October this year, 69 innovative drugs have been approved for listing in China, exceeding the total of 48 in last year, reaching a record high.
The higher the efficiency of examination and approval, the more drugs will participate in the competition, but the budget and payment capacity of medical insurance will not expand simultaneously. As a result, "much approval" directly translates into "difficulty in shortlisting", which forces supervision to further enter the value screening mode.
This is also the core background of this year's catalogue adjustment, which emphasizes "paying equal attention to both import and export".
In the "Frequently Asked Questions on Adjustment of National Basic Medical Insurance, Maternity Insurance, Work Injury Insurance Drug List and Commercial Health Insurance Innovative Drug List (2025 Edition)", it is clear that a monitoring mechanism should be established for drug allocation in the list, and for drugs with low clinical value, which can be replaced by other varieties, have not been produced and used for a long time, and cannot guarantee effective supply, priority should be given to transferring the basic list.
According to statistics, a total of 29 kinds of drugs have been transferred out of the catalogue this year, including both conventional catalogue products and 2024 negotiation catalogue products. Including:
Allisy Youzumab injection, a long-acting lipid-lowering drug PCSK9 inhibitor;
The controversial domestic drug for Alzheimer's disease, sodium mannate capsule 971, etc.
The former has withdrawn from the China market; The latter has been discontinued because the drug registration certificate has not been renewed.
In addition, Geli's hepatitis C drug danorevir sodium, Unacon's lymphoma drug Duweiliser capsule, and Renhui Bio-Benarutide injection (diabetes indication), the first GLP-1 drug in China, all appeared in this year's medical insurance catalogue, which failed to renew the contract.
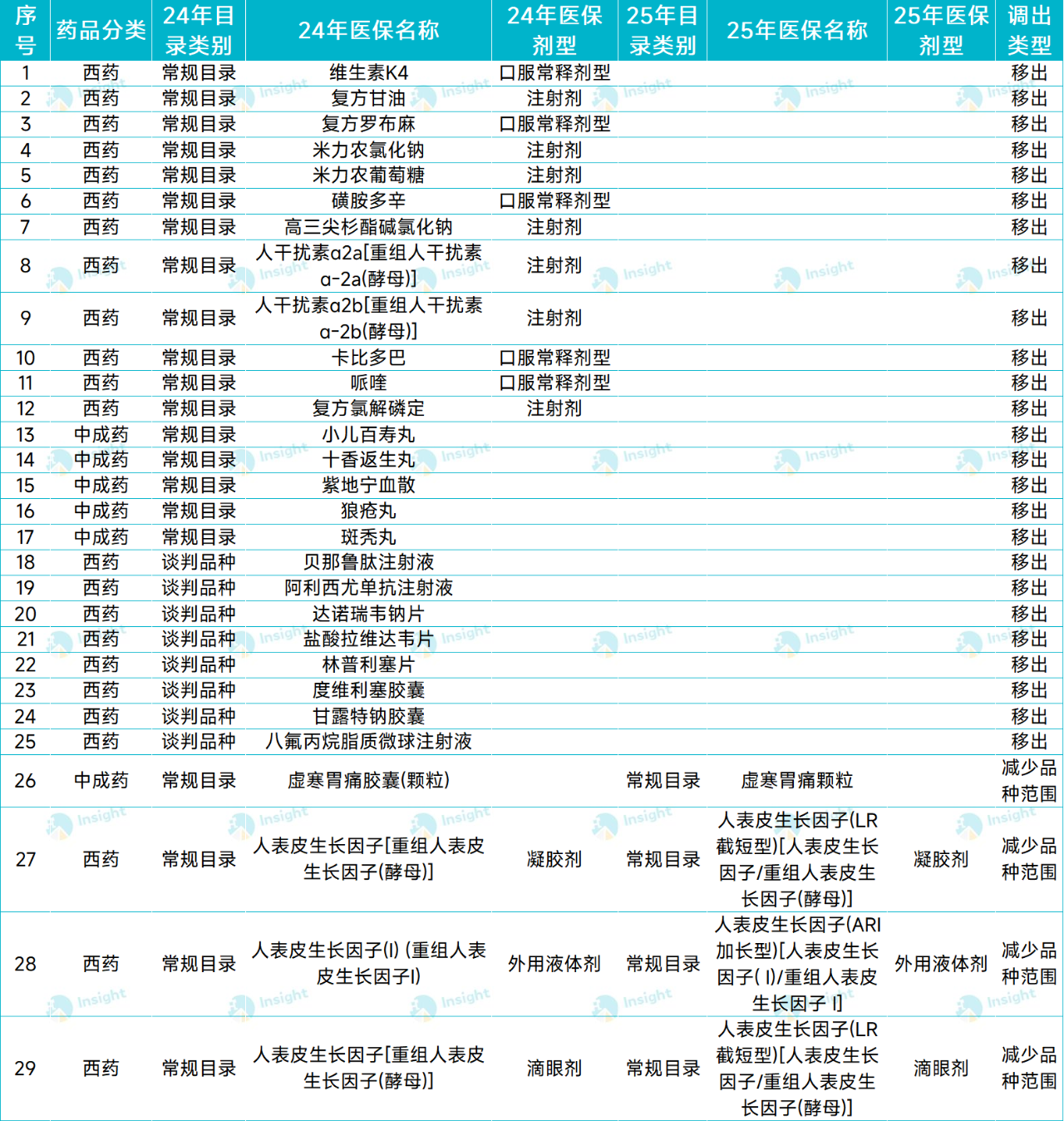
▲ List of 29 drugs transferred from 205 countries/collation of /Insight database.
In addition, there are nearly 300 players who have passed the examination of drug forms out of the catalogue, but are not qualified for negotiation. Among them, two new drugs for sleep disorders are "unexpectedly unsuccessful": Leibo Leisheng tablets from Weicai and Dali Leisheng tablets from simcere.
What the losers have in common is that there is either insufficient evidence of value, huge payment pressure or different business strategies.
02
"value promotion" of foreign enterprises and "quantity explosion" of domestic enterprises
Different from the "systematic retreat of foreign companies" in centralized procurement, the medical insurance catalogue has always been a strategic highland for innovative pharmaceutical companies. Whether domestic or imported.
In terms of foreign companies, more than 30 new drugs were included in the catalogue this year, continuing the "value-oriented" route of multinational pharmaceutical companies as a whole, especially the three major sectors of chronic diseases, tumors and self-exemption.
AstraZeneca performed the best, with three innovative drugs entering the medical insurance catalogue for the first time, eight products adding indications or renewing contracts, and changing to routine. The newly-added products are Quankede (Carpisette Tablets), Kangkeqi (Arctinib Maleate Tablets) and Fanshuzhuo (Benrellizumab Injection).
Roche has three innovative drugs for the first time this year, and seven products have added indications or renewed their contracts. Newly added products: Ixilai (Inalisexed Tablets), Gaoluohua (Gefitomazumab Injection) and Rococos (Aurelizumab Injection).
Other foreign companies have also steadily promoted important new products:
Pfizer: Siphon is included in the basic medical insurance, and Ireo is included in the catalogue of innovative drugs of commercial insurance;
BMS: Liblau Zexin, a red blood cell maturation agent, entered the medical insurance, which is the first approved treatment for low-risk myelodysplastic syndrome (MDS) in recent 20 years.
Novartis: 2 new drugs +4 indications were successfully included, and both inksland sodium and bustuzumab were "heavy potential stocks";
Sanofi: Ashatoximab was included in medical insurance less than one year after its listing, reflecting the "China speed";
GSK: Newly added indications of CRSwNP into medical insurance, realizing full medical insurance coverage of EGPA, SEA and CRSwNP.
It is worth noting that Lilly's new "global drug king" telpotide was included in the national medical insurance for the first time, which is suitable for the blood sugar control of adult patients with type 2 diabetes, which has aroused great concern in the industry. Dehelan, vice president of Lilly Group and general manager of China, delivered a speech on the spot as a representative of multinational pharmaceutical companies.

▲ Dehlan gave a speech at the conference.
In terms of domestic companies, head companies broke out in a concentrated way, and this year's momentum is even stronger.
Hengrui became the "biggest winner" this year. A total of 20 new drugs/indications were selected, of which 10 entered the medical insurance for the first time, which obviously entered the "commercialization cash cycle", and HER2-ADC, JAK1 and other potential large varieties blossomed in an all-round way.
Cinda Bio-limited, 7 innovative drugs/new indications were included in medical insurance this year, 6 of which were included in medical insurance for the first time, including a self-developed bio-similar drug IGF-1R monoclonal antibody for thyroiditis.
It is also worth noting that:
Kangfang Bio: Five innovative drugs have fully entered or expanded medical insurance, covering a number of first-line heavy indications, and IL-17A monoclonal antibodies are expected to be approved in the first half of 2026 and will be discussed at the end of the year;
Columbotai: In the ADC harvest period, the indications of Lucasatuzumab expanded rapidly and started to advance to the first line, and HER2-ADC and RET inhibitors continued to improve the product matrix;
Baekje Shenzhou: This year, the renewal of the contract is the main thing, and the new auxiliary indications for lung cancer are included in the medical insurance. The new Bcl-2 Sotok pull tab is expected to be approved in the first half of 2026, or it will compete with Yasheng;
Connaught: The three major indications of IL-4Rα are in the catalogue, and in 2026, it will face direct competition with Sanofi's medical insurance for the first time.
In addition, Fosun, Beida, Alice, Zhixiang Jintai, Haichuang Pharmaceutical, etc. have a number of new products that are first introduced or adjusted, covering many fields such as cancer, infection, chronic diseases, ophthalmology, etc., which reflects the dual support direction of medical insurance for innovation value and clinical needs.
03
In the first year, the catalogue of commercial insurance was "cautious"
In 2025, the price negotiation of commercial insurance drugs finally landed. The National Medical Insurance Bureau completed the formulation of the catalogue of commercial insurance innovative drugs in the first year by "crossing the river by feeling the stones"-121 innovative drugs passed the formal review, and only 19 of them finally entered the catalogue, far below the average volume of 41 Huimin special drugs.
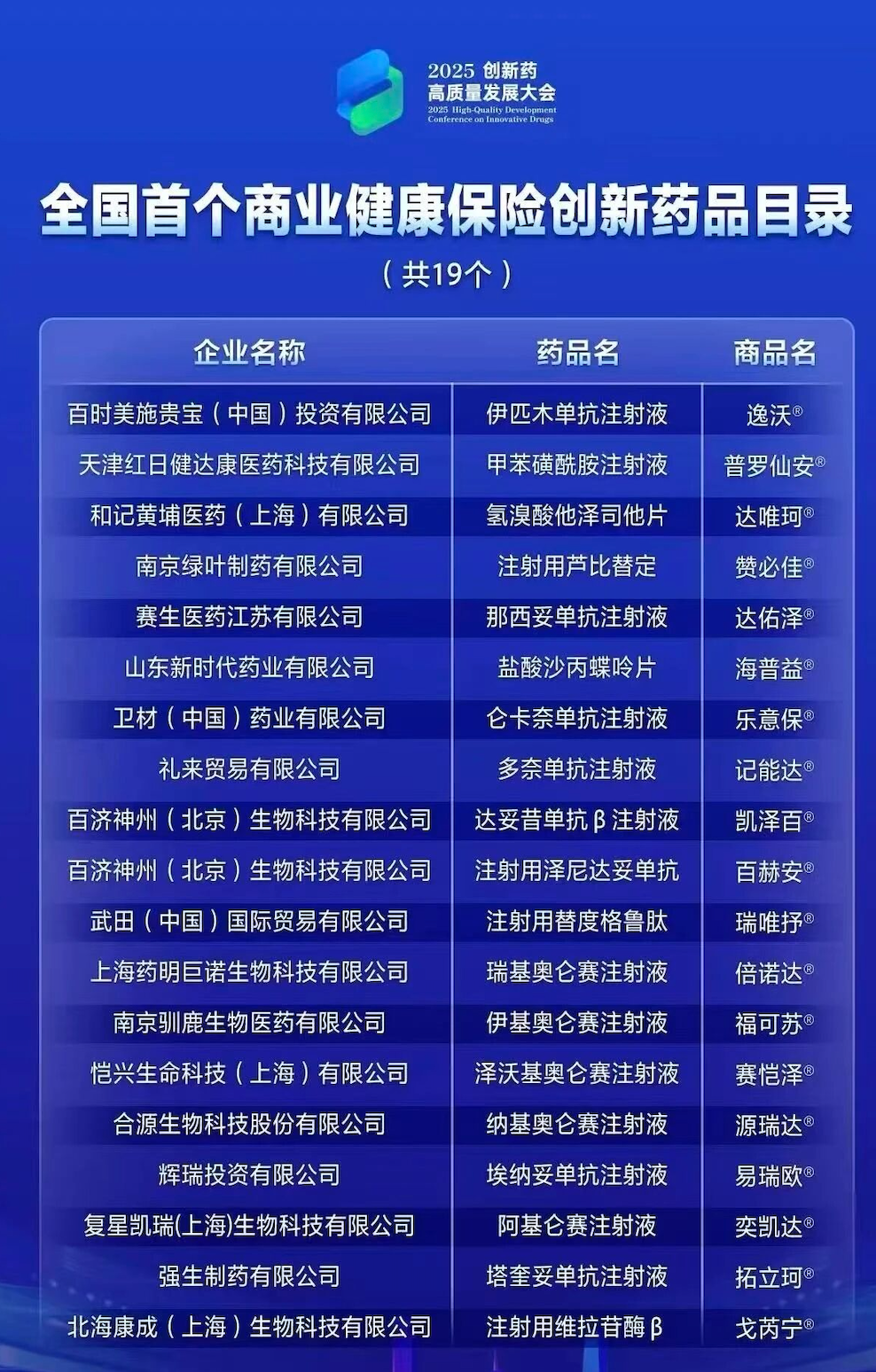
Overall, the first edition of the Catalogue of Commercial Insurance Innovative Drugs has three points:
First, all five domestic CAR-T models are listed, forming the strongest product cluster.
Five models of "Million CAR-T" from Fosun Kerry, Yaoming Junuo, Heyuan Bio, Reindeer Bio and Keji Pharmaceutical (Kaixing Life Science and Technology) were selected by the whole army, showing the strong signal of "deterministic efficacy+high-value innovation" in medical insurance negotiation.
However, the expected increase in the volume also indicates that the competition will further heat up in the future.
Second, cancer drugs are still the absolute main battlefield, accounting for more than 70%.
Of the 19 drugs, 14 are tumor drugs, and the most representative one is epimuzumab:
It was listed in China in 2021, and its indications cover a variety of solid tumors;
In 2024, the global sales reached US$ 2.53 billion, making it the most commercialized product in this catalogue.
Third, the "chronic disease+rare disease" double breakthrough.
Two new AD drugs of global concern, lencainidemab (Weicai) and donemab (Lilly), were selected at the same time. The former has a strong volume shortly after its listing in China, and its sales in the first half of 2025 has exceeded 50 million yuan.
From the perspective of enterprises, foreign pharmaceutical companies Takeda, Pfizer, Johnson & Johnson and Lilly all have products selected, while domestic companies Beihai Kangcheng, New Era Pharmaceutical, SciClone Pharmaceuticals and Baekje Shenzhou have also achieved breakthroughs.
Some experts have commented that the entry of rare and chronic drugs into the commercial health insurance catalogue is essentially a breakthrough in the boundary of the traditional commercial insurance risk model. "In many countries, such drugs are the categories that commercial insurance is the most afraid to touch."

04
The attitude of enterprises towards commercial insurance;
Love, doubt, and have to play.
However, from "listing" to "landing", the industry generally feels not only excitement, but also collective questioning about "boundaries"-the boundaries of basic medical insurance, commercial insurance responsibilities, product design, and local payment ability ... These will determine the upper limit of imagination of the future commercial insurance catalogue.
Look at the market scale first. The 9 trillion plate of commercial health insurance has stimulated the industry for a whole year, but the gap between reality and ideal has awakened everyone.
The White Paper on Multi-payment of Innovative Medicines in China (2025) shows that in 2024, the domestic sales of innovative medicines reached 162 billion yuan, and the commercial health insurance expenditure was about 12.4 billion yuan, accounting for only about 7%.
According to the relevant person in charge, Huimin Insurance, which has high hopes, only contributed 1.1 billion to the payment of innovative drugs last year.
In addition, according to the analysis of relevant experts, as of the end of July this year, a total of 313 Huimin insurance products were launched nationwide, and only 9 Huimin insurance products were added. Although there is a merger of provincial-level and municipal-level Huimin insurance products, it has obviously slowed down.
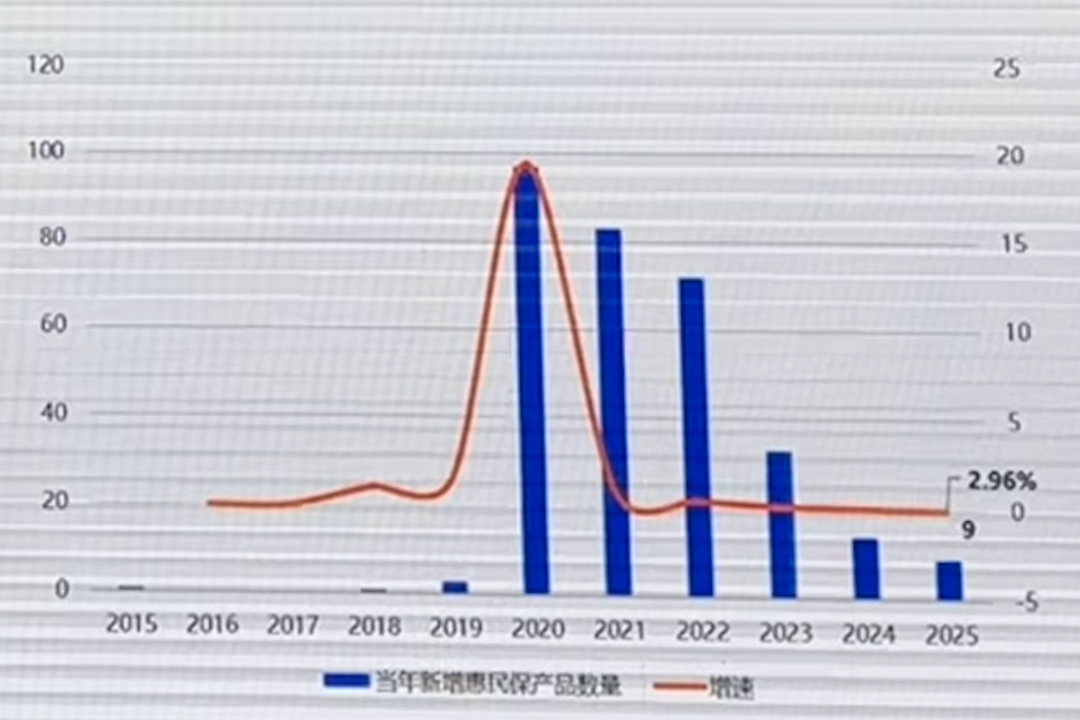 picture
picture
▲ Quantity and growth rate of new Huimin package products in that year
In essence, the full closed-loop construction of Shangbao's innovative drug list from "list release" to "patient benefit and industrial blood return" is a systematic project covering payment model design, data support, medical coordination and policy environment optimization.
Although the National Medical Insurance Bureau has put forward the "three exclusion policies" for the first time, it still needs the coordination of medical insurance, medical care, medicine and finance. According to the consensus of industry discussion, the "blocking points" include:
The responsibility boundary of the participants is vague;
The local medical insurance ability is different, and the undertaking ability is uneven;
Most commercial insurance products don't cover past diseases, so patients can't benefit. ......
Behind this, many business people's attitude towards this new thing is: thank the Medical Insurance Bureau for its support and hard work on innovative drugs, but the geometric effect really needs everyone to explore and promote together.
In the era of tight balance of medical insurance, everyone hopes that the catalogue of commercial insurance can resist the burden of the development of innovative drugs. People are full of expectations, and the hope of landing is flickering, but no one can tell how far it is from that beam of light.
Perhaps, in this era, there is no such shore.
Source: Source: MedTrend Medical Trends
·END·
相关推荐
- 2026 Guangzhou Medical Expo Scheduled for August! Building the Core Engine for the Greater Bay Area's Healthcare Industry. 2025-11-07
- Full-chain Empowers Industrial Innovation! The 2025 Guangzhou Medical Expo Successfully Concludes Today 2025-08-24
- Grand Opening of 2025 Guangzhou Medical and Health Industry Expo: Jointly Paint a New Blueprint for Health and Play the March of Industrial Endeavor 2025-08-22
- Over 400 hospitals and enterprises gather in the City of Rams! Guangzhou Medical Expo has become a highland for the first launches of innovative pharmaceuticals and medical devices. 2025-06-13

