Company Introduction:
The Guangzhou Innovative Drug Clinical Trial Service Center is an innovative service institution established by the Guangzhou Municipal Health Commission to promote communication and services among pharmaceutical innovation enterprises, clinical trial institutions, and clinical researchers in accordance with the spirit of the Guangzhou Municipal Government's Several Regulations on Accelerating the Development of the Biomedical Industry. It is the first government led new and integrated innovative drug clinical trial service platform in the province. The Service Center Management Office is located in the Municipal Health Commission, and the executing agency of the Service Center, Guangzhou Biotechnology Center Co., Ltd., is responsible for the specific administrative and public service functions of the Service Center.
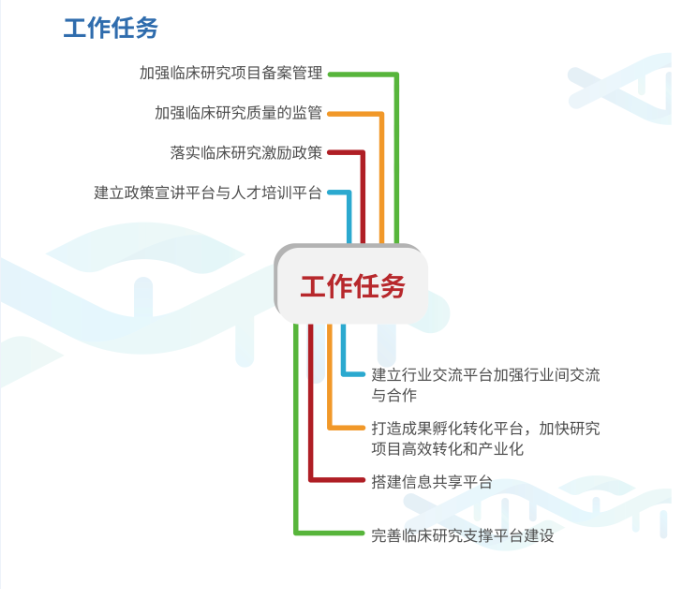
The service center aims at clinical trials of registered drugs, medical devices, hospital preparations of medical institutions, post marketing secondary development of traditional Chinese patent medicines and simple preparations, diagnostic reagents, etc., as well as clinical research initiated by researchers to create clinical research project filing management, strengthen clinical research quality supervision, implement clinical research incentive policies, establish policy publicity and talent training, industry exchanges, results incubation and transformation, information sharing We strive to achieve the development goals of leveraging the advantages of clinical medical resources in Guangzhou, improving the efficiency of clinical research resource utilization, building a good pharmaceutical innovation and research and development link, and building a highland for clinical trial aggregation through clinical research support and other platforms as soon as possible.
Service center five-star recommendation service:
Project Inspection:
The service center cooperates with the Drug Clinical Trial Professional Committee of Guangdong Pharmaceutical Association, supported by a team of experts from the committee, to provide clinical trial project inspection services to pharmaceutical equipment enterprises, CROs, and clinical trial institutions.
Patient Recruitment: Relying on abundant clinical doctor resources, we provide professional clinical trial recruitment services for enterprises and research institutions.
Medical enterprise docking:
By organizing special events, industry seminars, technical exchanges, training and other activities, we assist GCP institutions in multi-level communication and cooperation with target customers such as enterprises, promote communication with enterprises, and expand project sources.
Medical device to worker conversion:
The Guangzhou International Medical Device Medical Transformation Center provides one-stop, full process technical services for the incubation and transformation of regional medical device achievements, builds a new industry ecosystem of "public platform+medical device cloud+professional services", and helps the high-quality development of the medical device industry.
Official account operation:
Provide GCP with services such as WeChat official account hosting, WeChat official account business function development, WeChat applet development and customization, WeChat official account promotion, video account content production and operation.